Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliation: Charité Universitätsmedizin Berlin, Berlin, Germany (M. Piche-Ovares, A. Moreira-Soto, C. Fischer, S. Brünink, J.F. Drexler); Instituto Nacional de Salud, Lima, Peru (M.P. García Mendoza, M.D. Figueroa-Romero, N.S. Merino-Sarmiento, A.I. Marcelo-Ñique, C.A. Cabezas Sanchez); Universidad Nacional de Costa Rica, Heredia, Costa Rica (A. Moreira-Soto); Universidad Peruana Cayetano Heredia, Lima (E. Málaga-Trillo); Laboratorio de Refencia Regional en Salud Publica, Loreto, Peru (M. Gatty-Nogueira); German Centre for Infection Research, associated partner of Charité-Universitätsmedizin Berlin, Berlin (J.F. Drexler)
The Venezuelan equine encephalitis antigenic complex of alphaviruses encompasses 6 subtypes (I–VI), originally designated according to antigenic properties (1); those 6 subtypes are further divided into antigenic varieties. Venezuelan equine encephalitis virus (VEEV) consists of 5 varieties (A, B, C, D, and E); subtype ID is enzootic in Central and South America (2). The VEEV ID transmission cycle involves mosquitoes of the genus Culex (subgenus Melanoconion); however, knowledge of vertebrate sylvatic hosts is scarce (3). A prior study from the Peruvian Amazon conducted in 2001–2007 using molecular and serologic techniques indicated that up to 7% of febrile cases are potentially caused by VEEV, making it one of the most relevant arthropodborne viruses regionally (4).
VEEV has been detected continuously in humans in the Peruvian Amazon since 1993 (4,5). In 2006, a reported outbreak of VEEV resulted in a 5-fold increase in cases (63 cases) compared with previous years (10–14 cases/year) and 2 human deaths (6,7). All samples sequenced in the 2006 outbreak belonged to subtype ID (8). Since 2013, no data have become available on VEEV from Peru, so it is possible that the epidemiology of VEEV might have changed. We thus investigated VEEV in the Peruvian Amazon during 2020–2021 by using molecular, serologic, and bioinformatic methods.
We screened 1,972 serum samples from febrile humans sampled in the Peruvian Amazon as part of routine acute febrile illness surveillance in 2020–2021 during overlapping dengue virus and SARS-CoV-2 outbreaks (9). We tested samples for alphavirus RNA by a genuswide, nested reverse transcription PCR (RT-PCR) (10). Two serum samples (0.1%, 95% CI 0.001%–0.4%) tested positive for the presence of VEEV RNA. We obtained the first sample (Peru_2020) from a 74-year-old man from the district of Nauta in February 2020 (Figure 1). The patient sought treatment for headache, fever, muscle pain, and respiratory symptoms 4 days before sampling. The patient’s viral load was 8.1 × 105 copies/mL, as determined by a strain-specific real-time RT-PCR (6), consistent with the time elapsed since the onset of symptoms. We obtained the second sample (Peru_2021) from a 12-year-old girl in January 2021. Her viral load was 1.2 × 103 copies/mL, but no additional information was available. Isolation of both samples on Vero cells enabled the viral genomes to be characterized by high-throughput sequencing (Illumina, https://www.illumina.com) (Appendix).
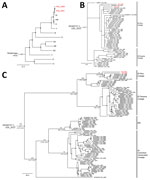
In a Bayesian whole genome–based phylogeny, the 2 newly generated sequences (GenBank accession nos. PP700505 and PQ513527) clustered with the ID subtype (Figure 2, panel A). We also observed clustering within ID in phylogenies based on all publicly available envelope glycoprotein precursor genomic sequences of the ID Panama/Peru lineage (Figure 2, panel B). Sequence comparisons indicated that both VEEV strains from our study were closely related (sequence identity 97.6%–98.1%) to other Peruvian strains belonging to the ID lineage but not were monophyletic, highlighting the cocirculation of different strains and suggesting maintenance of that VEEV clade in the region. We estimated the time to most recent common ancestor at ≈54 years ago (95% highest posterior density 38–66 years ago) (Figure 2, panel C) and noted evidence for purifying selection that suggested a regionally stable VEEV transmission cycle (11) (Appendix).
To determine past VEEV infections in Peru’s Loreto department, we selected a subset of 463 samples collected during January–April 2020 and September 2020–January 2021, corresponding to rainy seasons, when vector presence is highest and transmission is most likely. Samples were also selected to be negative for dengue virus by RT-PCR and of sufficient volume to enable serologic testing. We tested serum samples first by plaque reduction neutralization test (PRNT) in a 96-well plate format at 1:40 serum dilution to neutralize ≈50% (PRNT50) of the plaque-forming units of a VEEV isolate from this study. We retested positive samples (53/463) by PRNT50 in a 12-well plate format, which enables better count of plaque-forming units, in 2-fold dilutions ranging from 1:40 to 1:320 (Appendix). The titration showed that 18 of the 463 samples (3.9%, 95% CI 2.4%–6.1%) robustly demonstrated neutralizing antibodies (Table 1). Of note, a 2006 study reported a VEEV seroprevalence of 23%, which differed from our results for unknown reasons, potentially including sampling focused on urban areas and the VEEV outbreak reported during that year (7).
Considering the potential for cross-reaction with other alphaviruses, we tested PRNT50–positive samples by using a commercially available indirect immunofluorescent assay (IFA) (EUROIMMUN, https://www.euroimmun.com). The assay was based on cells infected with VEEV along with chikungunya, o’nyong-nyong, Ross River, Western equine encephalitis, Sindbis, Barmah Forest, and Eastern equine encephalitis viruses, as well as virus-like particles for Mayaro and chikungunya viruses (Appendix). Serum samples were tested at 1:100 for IgG and at 1:10 for IgM, which is the serum dilution recommended by the manufacturer. All PRNT50–positive serum samples tested positive for VEEV in the IgG IFA, and we observed monotypic VEEV reactivity in 9 of 18 samples (50%, 95% CI 29.0%–71%) (Appendix Figure 1). All other samples reacted with ≥2 viruses, results expected in tropical areas where several alphaviruses cocirculate (12). Cross-reactivity might have affected IFA patterns, since reactivity was observed with viruses not previously reported in Latin America, such as o’nyong-nyong, Ross River, Sindbis, and Barmah Forest viruses (13) (Table 1). Reactivity patterns suggested a predominance of Mayaro (n = 7) over chikungunya (n = 5) virus and of Western equine encephalitis (n = 6) over Eastern equine encephalitis (n = 2) virus, highlighting the relevance of targeting Mayaro and Western equine encephalitis in regional diagnostic testing and targeted epidemiologic studies.
IgG detection rates did not vary significantly by sex (p = 1.0 by Fisher exact test) but were higher in adults >30 years of age (p = 0.0001 by Fisher exact test), potentially a consequence of the 2006 VEEV outbreak (Table 2) (7). Adapting the IFA for IgM yielded entirely negative results, which is consistent with low levels of VEEV-specific IgM in the serum of acutely infected patients (14) and predominant detection of nonrecent infections in this study (Appendix Figure 2).
Despite evidence for the medical relevance of VEEV ID in the Peruvian Amazon from a prior study (5) and our study, the diagnosis of VEEV is not included in the routine arbovirus surveillance panel of Peru (N°125-MINSA/2016/CDC-INS). Our study demonstrates that the lack of surveillance and diagnosis is making the infection go unnoticed.
In tropical regions, factors such as the proximity of the rainforest to urban areas, agricultural activities, and deforestation may increase the likelihood of human contact with vectors, leading to virus transmission (14). These factors are of particular significance in the Peruvian Amazon, where ≤96.7% of households engage in activities related to agriculture or aquaculture (https://data-peru.itp.gob.pe). This information could explain why adults are more likely to be in contact with the virus and why nonpharmaceutical interventions to combat the spread of SARS-CoV-2 do not appear to affect the transmission of VEEV (15).
Beyond increased diagnostics, future research should prioritize investigating transmission cycles of different VEEV subtypes to identify populations at risk and focus on potential prevention strategies, such as targeted control of vectors and identifying potential amplifying vertebrate hosts (1). Arboviral surveillance, including of VEEV, should be generally strengthened with a syndromic diagnostic approach, particularly during periods of increased rainfall and harvest season, when the risk of arboviral infection may be increased (14).
Ms. Piche is a PhD student at the Institute of Virology at Charité-Universitätsmedizin, Berlin, and the Institute for the History of Medicine at Justus Liebig University of Giessen. Her research is focused on the epidemiology of arthropodborne viruses.