Mycoplasma species belong to the class Mollicutes, a group of bacteria characterized by lack of a cell wall. Those organisms are among the smallest organisms capable of autonomous replication and measure ≈0.3–0.8 μm.
M. arginini is a common colonizer in respiratory and urogenital tracts of various animals, including cats, dogs, cattle, and sheep, and is generally considered to be of low pathogenicity (1). However, in immunocompromised persons, M. arginini infection can lead to severe complications (2–4; M.A. May et al., unpub. data, https://www.preprints.org/manuscript/201809.0533/v1). We report a case of M. arginini infection in a kidney transplant recipient in Slovenia.
A 56-year-old woman was seen for a 3-week history of swelling, redness, and pain in her left forearm. She had a 17-year history of IgG kappa plasmacytoma and C3 glomerulonephritis, which led to end-stage renal failure and 2 kidney transplants. She underwent her first kidney transplant in 2011 and then a second transplant in 2022 after failure of the first graft. Both transplants were complicated by C3 glomerulonephritis recurrence. Approximately 6 months after the second kidney transplant, she started treatment with high-dose intravenous methylprednisolone, rituximab (chimeric monoclonal antibody targeting CD20 on B cells), and plasmapheresis, in addition to standard immunosuppressive therapy (tacrolimus, mycophenolate mofetil, and methylprednisolone). During that intensive immunosuppression, opportunistic infections developed, including cutaneous Alternaria alternata fungi infection of the left shin and cytomegalovirus reactivation, which necessitated adjustments to her immunosuppressive regimen. Over the next 2 years, declining graft function caused by C3 glomerulonephritis was managed with ravulizumab (a monoclonal antibody targeting complement component 5) and plasmapheresis. Granulocyte colony-stimulating factors were intermittently administered to treat episodes of neutropenia.
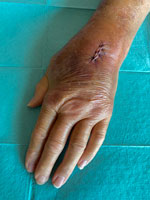
Approximately 2 years after her second kidney transplant, she noticed a small lump in the left mid-forearm with redness that progressively spread toward her wrist and elbow. Her doctors prescribed oral amoxicillin/clavulanic acid. After 5 days, that treatment failed to resolve her worsening symptoms, which prompted hospitalization. At admission, she exhibited swelling, redness, and restricted joint mobility in the left wrist. The patient had frequent contact with household cats and a dog and reported having sustained a cat bite at the site of small lump in the mid-forearm a week before admission.
Laboratory findings showed elevated C-reactive protein and β-d-glucan levels, normal procalcitonin level, and normal leukocyte count. Immune status evaluation using QuantiFERON Monitor (QIAGEN, https://www.qiagen.com) showed a moderate cell-mediated immune response, elevated plasma Torque Teno virus DNA (167,000 copies/mL), substantially decreased B-lymphocyte counts (likely caused by rituximab), and severe hypogammaglobulinemia (Table; Appendix). An ultrasound confirmed cellulitis, tenosynovitis of carpal extensor tendons, and arthritis of the radiocarpal joint (Appendix Figure 1).
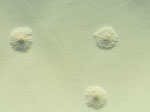
At admission, doctors initiated intravenous flucloxacillin therapy. The next day, doctors added doxycycline after an atypical infection was suspected. Surgical intervention on the radiocarpal joint included irrigation and the collection of synovial fluid and a biopsy specimen for microbiological analysis (Figure 1). Broad-range bacterial PCR with sequencing from synovial fluid identified M. arginini with 99.5% sequence identity; therefore, the sample was plated on arginine-enriched A8 agar. After 4 days, stereomicroscopy showed small colonies with a characteristic fried egg appearance (Figure 2).
After identification of Mycoplasma spp. from synovial fluid, we discontinued flucloxacillin and added moxifloxacin. The combination of doxycycline and moxifloxacin led to clinical improvement that allowed de-escalation to doxycycline monotherapy after 8 days. We treated severe hypogammaglobulinemia (IgG 2.7 g/L) with intravenous immunoglobulin on day 10 after admission. We discontinued mycophenolate on day 6 after confirmation of M. arginini infection. Ravulizumab, last administered 6 weeks before hospitalization, was postponed because of ongoing infection. Redness, swelling, and pain in the wrist and elbow resolved, and we discharged the patient on a 10-week course of doxycycline with outpatient follow-up.
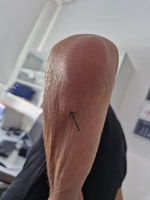
After discharge and 3 weeks of doxycycline treatment, we excised a painless subcutaneous nodular lesion below the left elbow (Figure 3). Broad-range PCR performed on a sample from lesion confirmed M. arginini with 100% sequence identity. Mycoplasma spp. culture was negative. Histopathology showed mild inflammatory changes consistent with bacterial infection. Four months after ending the 10-week course of doxycycline treatment, the patient was doing well, without any local or systemic signs of M. arginini infection.
We tested oropharyngeal swab specimens from the patient’s 3 cats and 1 dog using a genus-specific mycoplasma PCR (5) and confirmed colonization with M. gateae and M. maculosum. We found that 1 cat had a mixed Mycoplasma infection, but we could not definitively confirm or rule out M. arginini in that cat. We gave a 2-week doxycycline course to the pets to treat Mycoplasma, although reinfection remained possible because they moved indoors and outdoors.
Only a few documented published cases support zoonotic potential of M. arginini, particularly in immunocompromised patients. Those cases include reports of disseminated infection in a slaughterhouse worker with advanced non-Hodgkin lymphoma (2), septicemia with polyarthritis in another therapy-resistant non-Hodgkin lymphoma patient who had close contact with several cats (3), a disseminated infection in a young bodybuilder with a history of use of nutritional supplements derived from animal materials of uncontrolled origin (6), and a deep tissue infection in a hunter with an open femur fracture from a lion attack (7). Common among those cases were profound immunosuppression and consumption of animal products or close contact with animals, as seen in our patient.
Research indicates that M. arginini poses little risk to persons with healthy immune systems. A study of 22 persons at occupational risk for Mycoplasma infections (e.g., veterinarians, farmers, and slaughterhouse workers) found no substantial infection risk in those with normal immune function, suggesting that human exposure to M. arginini is generally not a clinical concern for immunocompetent persons (8). Most documented cases, including the patient we report, involve immunocompromised persons, often with hypogammaglobulinemia, which is a known risk factor for infections caused by Mycoplasma spp. (4).
The optimal treatment for M. arginini infection remains uncertain because of its rarity in humans. Reported cases are typically managed with long-term courses of antimycoplasmal antibiotic drugs, such as macrolides, quinolones, and tetracyclines (3,6,7). In 1 instance, a patient treated with erythromycin ultimately died from the infection, and postmortem testing showed erythromycin-resistant M. arginini (2). That testing suggests that broader-spectrum antibiotic drugs may be more effective as initial treatment, particularly because erythromycin resistance is well documented in several Mycoplasma species (9,10).
Because Mycoplasma bacteria lack a cell wall, infections are best addressed with intracellular-acting antibiotic drugs such as tetracyclines or macrolides. Dual antibiotic drug therapy has been applied in specific cases to improve treatment outcomes, especially in immunocompromised persons or patients with disseminated infections (11). Treatment duration remains unclear. A meta-analysis of septic arthritis caused by Mycoplasma spp. reported therapy durations ranging from 2 weeks to >2 years. Clinical improvement, normalization of inflammatory markers, and negative reculture results should guide discontinuation (11).
The case we report also raises questions about persistently elevated β-d-glucan levels, which decreased but did not normalize after treatment. Although Mycoplasma species are not commonly associated with β-d-glucan production, studies suggest some species, such as M. agalactiae, might produce it (12). The zoonotic aspect is also notable, considering the well-documented colonization of domestic animals by M. arginini and the patient’s history of cat bites, likely making her pets the source of infection.